Jim Booth, Adrian Goldberg & Nazrin Wilkinson
File On 4 Investigates

 BBC
BBC
Debbie Booker's knee implant led to serious and ongoing health problems
A knee-replacement implant, used in thousands of UK operations, was known to have a concerning failure rate eight years before it was finally withdrawn, the BBC has discovered.
Patients have told File on 4 Investigates how they were left immobile or addicted to painkillers after receiving the NexGen knee implant, because it ended up slipping out of place. Hundreds of people have now had to undergo a second corrective operation.
Knee surgeons say the implant's US manufacturer, Zimmer Biomet, took too long to acknowledge there was a problem with one particular component.
Zimmer Biomet says patient safety is its "top priority" and that its products are approved in accordance with the relevant regulations.
Debbie Booker from Southampton had an operation to replace her left knee in 2016.
Although initially it appeared to have been successful, she started to experience severe pain a year later while on holiday in Majorca.
"I laid a bag of ice on my knee and for four days I had to do that every few hours because I was in agony," she says.
A knee replacement involves removing damaged surfaces of the femur (thigh bone) and the tibia (shin bone) and replacing them with artificial components.
Debbie says the pain resulted from the knee implant slipping from the tibia and wearing away the bone.
Over the next few months she says she became reliant on prescription painkillers: "I was on fentanyl and morphine. It took me a long time to come off of the morphine because I was addicted."
She has since had a second knee replacement, but the problems caused by the initial failed implant have caused long-lasting health problems, she says.
"It's put my whole body out of alignment, I walk with a limp," says Debbie. As a result, she is now awaiting a hip replacement.
Another patient, "Diana" (not her real name), had a knee implant fitted in 2021 which also slipped and started to wear away her shin bone, leaving her virtually immobile.
"The consultant told me every time I stood up, I was standing on a broken leg. It was absolute agony," she says.
Diana asked to be anonymous as she used to work in the NHS.
As part of their knee replacements, both Debbie and Diana had received a specific implant section, known as a "stemmed option tibial component", also known as a "tibial tray".
In broad terms, this section lacked a layer of plastic contained in earlier, well-regarded versions of the NexGen replacement knee.

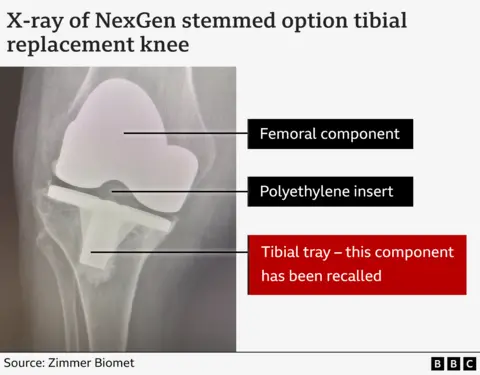
Zimmer Biomet started marketing this modified version in 2012. It was cheaper than the earlier model, so it made financial sense for the NHS, according to Prof David Barrett, a knee specialist at Southampton University.
"[The NHS] were justified by saying, 'we have every reason to think it'll be fine,'" he says.
In the decade that followed, more than 10,000 patients were fitted with this version of the implant.
However, File on 4 Investigates has discovered that concerns were first flagged in 2014 by the National Joint Registry (NJR) which keeps a record of implant surgery across England, Wales and Northern Ireland.
At that point, there was insufficient data to draw any reliable conclusions, the NJR told us. It is not an easy task to isolate a specific component that is not working as it should, it added.
Further concerns about the implant were raised in Ireland two years later, in 2016, by Prof Eric Masterson, a knee surgeon in Limerick.
Prof Masterson's corrective-surgery rate had soared after he started using NexGen implants in 2012 and he found his professional competence being called into question.
"That was a lonely place," he tells File on 4 Investigates. "You spend a lifetime building up a career and a reputation, and it's very easy to have that career shredded."
When he raised questions with Zimmer Biomet representatives, they assured him there wasn't a widespread problem, he says - an account echoed by NHS surgeons who told us they had found themselves in similar situations.


Prof Eric Masterson said the failure rate of the implant started to impact his career and reputation
Prof Masterson asked to be put in touch with surgeons in the UK to compare notes. However, confidential internal company documents seen by File on 4 Investigates reveal the company was only willing to contact surgeons on his behalf if they were considered "friends of Zimmer Biomet" and "happy with their NexGen patients".
Zimmer Biomet failed to act quickly enough after the problem was identified, according to Prof Leila Biant, one of the UK's leading knee surgeons. She says concerns were raised by herself and other colleagues as far back as 2017.
"The issue is [the company's] initial reluctance to acknowledge a problem and to not really engage with a process to evaluate these patients until [Zimmer Biomet] got to a situation where they had to," she tells us.


Zimmer Biomet failed to acknowledge the problem quickly enough, says Prof Leila Biant
In 2022, the NJR estimated that patients were nearly twice as likely to need corrective surgery after receiving the NexGen implant, when compared with the average knee implant.
In the same year, Zimmer Biomet recalled any unused implants from the UK market.
Estimations of failure rates for the tibial tray component in this NexGen implant vary from 6% (twice as much as should be expected) to 19%, according to peer-reviewed academic studies.
In a statement, the company told the BBC: "Zimmer Biomet is committed to the highest standards of patient safety, quality, and transparency. When new data becomes available, we act appropriately, responsibly, and in accordance with applicable regulatory requirements."
All 10,000 patients fitted with the problematic implants should now have been recalled for a review by the hospitals where they had their initial operations. Hundreds have already had to have a second operation, and others are likely to follow as problems come to light.
The cost of rectifying the problem is not cheap. Each revision costs between £10,000 and £30,000 because the implant is very specialised, says Prof Barrett from Southampton University.
"Patients are in hospital for a lot longer and they require more support. So this is a very significant expense," he says.
As a result, the total bill is estimated to run into millions of pounds.
Zimmer Bionet did not respond when File on 4 Investigates asked if it would be contributing to the cost of these operations. However, we have seen a confidential company email, sent in 2022, telling sales staff to say that "Zimmer Biomet will not cover diagnostic, follow-up or revision costs up front".
NHS England told us it was "currently reviewing the case involving Zimmer Biomet NexGen knee implants".

 German (DE)
German (DE)  English (US)
English (US)  Spanish (ES)
Spanish (ES)  French (FR)
French (FR)  Hindi (IN)
Hindi (IN)  Italian (IT)
Italian (IT)  Russian (RU)
Russian (RU)  4 hours ago
4 hours ago























Comments